- Climate Change
- Posted
Methane & climate change

With the threats posed by anthropogenic climate change now accepted as a key international issue, efforts to curb carbon dioxide emissions are becoming manifest around the world in spite of – and even as a response to – the global recession. But any such efforts may be in vain if the focus on carbon dioxide distracts from the need to curtail methane emissions, as Richard Douthwaite explains
One of the things that attracted me to economics was that you did not have go very far into it before you reached the frontiers of knowledge and could do useful research. I can remember a lecturer at university pointing this out. He was right. There are many examples of people who have moved into economics after mastering some other discipline and come up with valuable insights after only a few years.
With science, on the other hand, the lecturer argued that so much was known that you had to study an obscure area for years before you could find a topic that was worth researching. How wrong he was. I’ve just spent a few days mugging up about methane's role in climate change and I’m shocked and surprised to find how little is known and consequently, in view of its importance as a greenhouse gas, how much research is urgently needed.
My surprise is heightened because methane is such a simple gas – just four hydrogen atoms and a carbon one – which I knew about before I was ten. I grew up in a pit village in Yorkshire where coal face methane could kill – and did. I can remember being taken to one of the collieries where my father worked to see the canaries which were taken down the mine to warn of the gas’s presence. The birds are very sensitive to the gas and keel over if a dangerous concentration builds up.
My father also showed me a Davy lamp, which was invented by Sir Humphrey Davy, one of the great early chemists, to provide light for miners in the days before electric lighting came in. The lamp has a lighted wick but the flame does not set off an explosion because a metal gauze prevents the temperature of the escaping fumes rising sufficiently to ignite a dangerous methane/air mixture. However, if you looked at the flame carefully, you could tell how much methane was in the air by the size of the blue fringe around its yellow heart. As result, Davy lamps continued to be used in mines until electronic sensors came in a few years ago. They are still kept in reserve.

The Davy lamp not only provided light for miners but also was used as an indicator of how much methane was in the air
Across the field from where we lived was a dark pool surrounded by willow trees. If you stirred the black mud on the bottom with a stick, big bubbles came up and my father said this was methane too and was the cause of the will-of-the-wisp that had featured in a bed-time story. A few days later I went back to the pond, caught some gas in a jam jar and managed to get it to burn.
Because it had featured in my childhood, when I started reading up about methane recently, I felt that pretty well everything about it must be known. Not at all. We know dangerously little about where it comes from, what it does in the atmosphere and where it goes.
Take its origins. Until recently, it was thought that it had only one biological source – archaea, single-celled micro-organisms that belong to a major division of life and are quite different from bacteria with which they were once confused. Many types of archaea can survive extreme environmental conditions, such as temperatures above 100oC or extreme alkalinity or acidity.
One branch of the archaea family are called methanogens because they produce methane. Over fifty different ones have been identified, some of which were found three miles under the Greenland ice sheet and others in the Utah desert. These get the energy on which to live from hydrogen. Other micro-organisms split the carbohydrates in plant material, like the willow leaves that fell to the bottom of the dark pond, into hydrogen and carbon dioxide. The methanogens then use the hydrogen to turn the carbon dioxide into methane. Most of them need anaerobic conditions such as the bottom of ponds or cows' rumens in which to work.
In 2005 however, two scientists, Frank Keppler and Thomas Röckmann who were working at the Department of Agriculture and Food Science in Northern Ireland at the time, collected 30 different kinds of tree leaves and grasses from tropical and temperate parts of the world and put them in small test chambers. “To our amazement,” they wrote in Scientific American two years later, “all of the various kinds of leaves and plant litter produced methane. Usually, a gramme of dried plant material releases between 0.2 and three nanograms [billionths of a gramme] of methane an hour. These tiny amounts were difficult to monitor even using our highly sensitive state-of-the-art equipment.”

A methane airborne mapper technique used by Keppler and Röckmann
The pair went on to find that living plants put out between ten and a hundred times more methane than detached leaves. The amounts were still tiny – which explains why no-one had noticed them before – but collectively they matter because they really add up over the world as a whole. Keppler and Röckmann calculated that between 60 million and 240 million tonnes of methane were being released by plants every year. This is 10-40 per cent of annual global methane emissions, a serious matter since methane is many times more potent as a greenhouse gas than CO2.
Confirmation of their find came shortly afterwards from Germany where a group in Heidelberg had satellite photographs they could not explain showing high concentrations of methane over tropical forests. When word got out, people around the world began to ring in to radio programmes asking if they should cut down their trees. Fortunately, a quick calculation showed that trees still had a beneficial effect and slowed down the rate at which the world warmed. “New forests to absorb carbon dioxide would far exceed the relatively small negative effect of adding more methane to the atmosphere,” Keppler and Röckmann write.
This newly-discovered methane source has not yet been added to the charts being used by most of those trying to develop policies to limit climate change. Figure one is typical. It shows that emissions are estimated to have increased from 233 million tonnes of methane a year in pre-industrial times to about 600 million tonnes today. If emissions from plants were to be included in the pre-industrial estimate, it might even double. Although the inclusion would also increase the current figure, it would mean that the increase in methane releases over the past 250 years has been much less than everyone has assumed.

Figure one: current and pre industrial methane emissions
Another surprising gap in our knowledge about methane is that we are not really sure about what its warming effect is in comparison with carbon dioxide. The reason for this is that methane breaks down quite quickly in the atmosphere while CO2 stays there indefinitely and, of course, has a warming effect for as long as it does. The standard assumption is that it stays there for 100 years.
If you Google the half-life of methane in the atmosphere – the length of time before half of any release has disappeared – you will find the most popular figure is seven years, closely followed by 8.5 and with other bids ranging up to 20 years. One reason for this uncertainty seems to be that most methane is broken down by combining with hydroxyl ions in the upper atmosphere to make CO2 and water. The hydroxyl ions are produced when the sun's rays split an ozone molecule (O3) and each of the three free oxygen radicals that result joins with a water molecule to make two hydroxyl (OH) ions. If there are plenty of hydroxyl ions about, the methane is quickly destroyed but if a lot of methane is suddenly released, the supply of hydroxyls gets exhausted and the methane lingers on, continuing its warming effect.
So methane's half-life is not a fixed number. This means that it is impossible to compare the warming effect that it will have in comparison with CO2. The Intergovernmental Panel on Climate Change used to say that it was 23 times worse if its warming effect was considered over 100 years but now it says that a more likely figure is 25.
Of course, as methane's half life may be seven or eight years, almost nothing will be left of any release today in a century's time. Methane has a massive short-term heating effect which quickly dies away. Accordingly, if we want to have a big near-term impact on the rate at which the world is warming, putting the emphasis on stopping methane releases rather than CO2 ones might provide the way. As figure two shows, man-made methane emissions currently outweigh natural emissions.

Figure two: natural and man-made methane emissions

Frank Keppler who, together with Thomas Röckmann, discovered that all living plants produce methane
Figure three shows the concentration of methane in the Irish atmosphere in comparison with that on the other side of the world. Both have a pronounced annual cycle and it is thought that this is due to the annual variation in the amount of ozone in the atmosphere. The more ozone there is, the more hydroxyls get made and the more quickly the methane is broken down.

Figure three: methane concentrations in Ireland and Tasmania
Can this explain another big methane mystery? You'll have noticed from figure three that the Irish and Tasmanian methane concentrations each oscillated about their own levels. Neither showed any sign that they were going up from 1996 until last year despite the fact that the amount of methane that humanity has been releasing is thought to have been increasing. Yet if you look at data for earlier years, you'll see that the concentration was rising rapidly. Why has the rise stopped? Where has the extra methane been going? The answers are: no-one knows. But could it be that, now less ozone is being destroyed by man-made chemicals like CFCs, there's more in the atmosphere for methane removal?
Emissions from the global herd of ruminants – cattle, sheep, goats and camels – contribute about a quarter of the methane production that is under human control. As a result, the UN's Food and Agriculture Organisation is very concerned that if a quick reduction in the rate at which the Earth is warming is required, these animals might be targeted for the chop. It joined forces with the International Energy Agency to look into the mystery over why the atmospheric concentration of methane was not increasing and the two bodies produced a report, Belching Ruminants: a Minor Player in Atmospheric Methane, earlier this year. The report concluded:
Since 1999 atmospheric methane concentrations have levelled off while the world population of ruminants has increased at an accelerated rate. Prior to 1999, world ruminant populations were increasing at the rate of 9.15 million head/year but since 1999 this rate has increased to 16.96 million head/year. Prior to 1999 there was a strong relationship between change in atmospheric methane concentrations and the world ruminant populations. However, since 1999 this strong relation has disappeared. This change in relationship between the atmosphere and ruminant numbers suggests that the role of ruminants in greenhouse gases may be less significant than originally thought, with other sources and sinks playing a larger role in global methane accounting.
The Department of the Environment in Dublin is also interested in methane-belching cattle and has been eyeing the national herd for the chop for some time. This is understandable since methane from the country's livestock makes up about 13 per cent of Ireland's total greenhouse gas output and a fifth of the emissions outside the EU's emissions trading system. The EU is insisting that these non-ETS emissions have to be reduced by 20 per cent by 2020 and, if they are not, Ireland will face a massive fine. Consequently, if the herd isn't reduced, much larger cuts will be necessary elsewhere. “It's cars or cows,” an official was heard to say last year when explaining what he thought the reduction options were.
My renewed interest in methane is a product of the Department's concern. In September 2008, Feasta, the Foundation for the Economics of Sustainability, won a three-year contract from the Department to explore what its policy options were, not just for methane but also for carbon dioxide releases from drained peat bogs and nitrous oxide emissions from the breakdown of nitrates in the soil. I'm managing the project on a half-day a week basis. The real effort is being put in by Corinna Byrne, a soil scientist at the University of Limerick, who has set up the Carbon Cycles and Sinks network (see http://carboncyclesandsinks.org/) so that everyone with an interest in greenhouse emissions from the way the land is used and abused can contribute to shaping policy on what to do about them. Our first report is due out soon. We hope to produce another next year if our funding is renewed.
What we've found is that there's not a lot that can be done to reduce the amount of methane cattle produce short of killing them. Methanogens are only some of the 500-1000 microbial species found in a cow's rumen and less than 10 per cent of the others – bacteria, protozoa, and fungi – which have been cultivated and described. The methane is the joint product of them all. It is fundamental to what a cow is.
Small reductions can be made by altering an animal's diet by giving it more oil and starch in its feed, but this reduces a benefit of keeping ruminants which is that they can turn cellulose and other material humans can't eat into things that they can. You can also dose it with polyethylene glycol to affect the way tannins are processed. Or you can send beef animals for slaughter earlier so that they don't release methane when they are putting on weight more slowly as they mature. But it is all pretty marginal. Dr Frank O'Mara, the new director of research at Teagasc, estimated in 2007 that if all the stratagems could be combined – and some may be incompatible or too costly to use – the most that could be achieved was a 5.7 per cent reduction in methane production.

Anaerobic digesters could provide farmers with additional income by turning methane output into biogas energy
The best thing that can be done at present is to stop methane being produced when the animals are kept in sheds and their slurry is stored. This amounts to 21 per cent of Ireland's agricultural methane emissions and could be largely eliminated if anaerobic digesters were installed so that methane production was maximised and the output was burned to produce energy. Selling biogas this way would give farmers an additional source of income.
If the national herd was reduced by 20 per cent to meet the EU target, the lost milk and meat production would be carried out elsewhere, most probably in a developing country not subject to climate restrictions. This could actually lead to a net increase in greenhouse emissions, particularly if forest had to be cleared to provide pasture. 80 per cent of the forest cleared in Brazil has been used for cattle rearing.
Since the problem is a tough one to crack, it's just as well that we think we've been able to make it go away. We've come to the conclusion that the methane released by cattle does not matter at all provided that the pasture on which they are kept is well managed. The reason for this is that the soil under carefully-grazed grassland can take in and lock away about half a tonne of carbon (the equivalent of about 2 tonnes of CO2) per hectare each year.
Averaged over its life, a bullock going for slaughter at 26 months will release about 67 kg of methane each year. How soon will it be before the warming effect of that methane has been nullified because a balancing amount of carbon dioxide has been absorbed by the grass in the pasture and taken down into the soil? How big is the balancing amount?
If we use the IPCC's figure that the warming effect of methane is 25 times that of CO2, the balancing amount would be 25 times 67 which equals 1,675 kg. This is less carbon dioxide than a hectare of pasture takes up in a year. If that's the case and one growing beef animal was kept per hectare, Irish pasture would be an emissions sink rather than a source and the country would need to cut its emissions less in other areas.
This seems too good to be true. In fact, the IPPC's conversion factor of 25 is inappropriate because, as we noted, it compares the heating effect of releases of the two gases over 100 years. It does not compare the warming effect of two constant stocks. We need to look at stocks because, if Ireland keeps its national herd at a constant size, the methane the herd produces will build up in the atmosphere until the rate at which it is being destroyed by hydroxyls is equal to the rate at which methane is being added. In other words, a stable stock of ruminates on Irish soil produces a stable stock of methane in the atmosphere. So the question we need to ask is how big would that stable methane stock be and how much CO2 would need to come out of the atmosphere to nullify its heating effect?
If the half-life of methane is about 8 years, the atmospheric stock produced by any animal will be 11.54 times its annual emission. Thus, if we use a figure of 100 kg per year per livestock unit (a mature animal or the equivalent in younger stock), we get a stock of 1,154 kg of methane. According to a Cambridge climate modeller (nobody in Ireland seemed to know the figure), the instantaneous heating effect of this amount of methane would be 23 times the same weight of CO2. In other words, we need the pasture to remove 23 times 1,154 equals 26,500 kgs of CO2 from the atmosphere to make each livestock unit global warming neutral. At 2 tonnes of CO2 per hectare per year and one livestock unit per hectare, that would take 13 years, a figure I find remarkably low.
Our policy advice will therefore be that, as most Irish pasture has been grazed for at least ten times the length of time required to make each beast climate neutral, the government should tell Brussels that the methane from the country's livestock should be excluded from the annual emissions figure that Ireland is being required to cut. Will Brussels agree? We've no idea, but in our view the livestock sector has already made its carbon contribution and will continue to make further contributions if it's allowed to do so. It should therefore continue on its present scale and continue to earn the country millions of euros from its sales in Europe every year.

Methane from Ireland’s livestock makes up about 13 per cent of Ireland’s greenhouse gas output
- Articles
- Climate Change
- Methane & climate change
- carbon dioxide
- emissions
- recession
- anthropogenic
- ruminants
Related items
-
 Much ado about nothing
Much ado about nothing -
 Property industry faces ‘triple threat’ from climate crisis
Property industry faces ‘triple threat’ from climate crisis -
 Irish Green Building Council launch event to promote sustainable building practices
Irish Green Building Council launch event to promote sustainable building practices -
 Ashden Awards winners showcase climate solutions
Ashden Awards winners showcase climate solutions -
 Decarbonising buildings “most important issue” – Climate Change Committee
Decarbonising buildings “most important issue” – Climate Change Committee -
 Techrete aims for net zero carbon by 2030
Techrete aims for net zero carbon by 2030 -
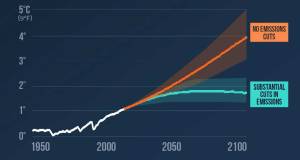 ‘Sufficiency’ key alongside energy efficiency & renewables, says IPCC
‘Sufficiency’ key alongside energy efficiency & renewables, says IPCC -
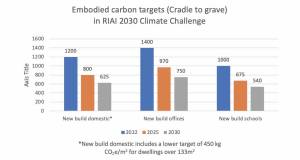 RIAI launches 2030 climate challenge
RIAI launches 2030 climate challenge -
 Climate action plan sets embodied carbon targets for construction
Climate action plan sets embodied carbon targets for construction -
 Grant invests in biofuel tech for oil boilers
Grant invests in biofuel tech for oil boilers -
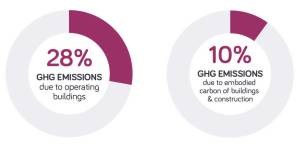 Architects call for urgent climate action ahead of COP 26
Architects call for urgent climate action ahead of COP 26 -
 The PH+ guide to overheating
The PH+ guide to overheating

